Water: the importance of analysis
The main parameters that need to be considered and monitored, with regard to a freshwater aquarium, are:
- pH
- hardness
- ammonia
- nitrites
- nitrates
- phosphates
pH is the measure of the water's acidity. It is measured on a scale ranging from 1 to 14. Low values (up to 7) correspond to acidic waters.
A pH value equal to 7 indicates neutrality, that is, a balance between the acidic components and the basic component (for the latter, the index varies from 7 to 14).
For freshwater aquariums, the ideal pH varies greatly depending on the type of plants and fish it supports, but ranges approximately from 5.5 to over 8.0. Most tropical fish prefer medium or slightly acidic values. However, even when faced with a (non-sudden) change in this value, fish are able to adapt without difficulty, so much so that, in nature, the same species can be found in aquatic ecosystems with very different values.
A pH check is recommended at every partial water change.
Carbonate hardness is measured in KH; its function in the aquarium is to stabilise the pH of the water by opposing any sudden variations in it. A KH value between 4 and 8 is generally suitable for most freshwater aquariums; values below 4 expose the tank to the risk of sudden changes in pH. For marine aquariums, KH values range from 8 to 10. The KH value also affects the amount of CO2 dissolved in the water and its assimilation by the plants. By knowing the KH and pH values of your aquarium, you can work out the amount of dissolved CO2, thanks to the relationships that regulate these three amounts (see the table below).
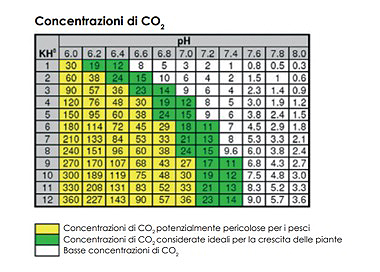
| Title: |
CO2 concentrations |
| Yellow: |
CO2 concentrations potentially dangerous for fish |
| Green: |
CO2 concentrations considered ideal for plant growth |
| White: |
Low CO2 concentrations |